Our Service
Center of Excellence for Consulting Clients in the Pharma and Healthcare Industry
Momentous Pharmaceutical Share Company (MPSC) has a dynamic center that delivers a high value pharma and healthcare advisory and consulting services designed to manage risk, improve performance, and achieve strategic objectives. The center leverages a multitude of world-class academic and biopharma investigators with innovative, experienced and well-established expert resources it possesses to assist with the operational, planning and transactional activities of governmental as well as non-governmental (for profit or not for profit) institutions considered as upstream drivers of the healthcare landscape.
MPSC Serve as center of excellence for commissioned research, training, testing and providing advice for governmental, non-governmental and private institutions involved in healthcare delivery.
Pharma Companies
Health Workforce Training and Certification
Contract Research
Technical Document Translation
Advisory Activities
Import and Distribution
Public, private and Non-Governmental Organizations are the major stakeholders involved in the sourcing and distribution of pharmaceuticals, medical supplies and laboratory reagents and medical equipment in Ethiopia (Fig 1). According to Frost and Sullivan, the annual pharmaceutical market for Ethiopia is estimated to grow each year by 14% and exceed more than one billion dollars by 2020. While development partners cover 40% of the finance for pharmaceutical expenditure, the rest are covered out-of-pocket (37%), by government (21%), and employer insurance schemes.
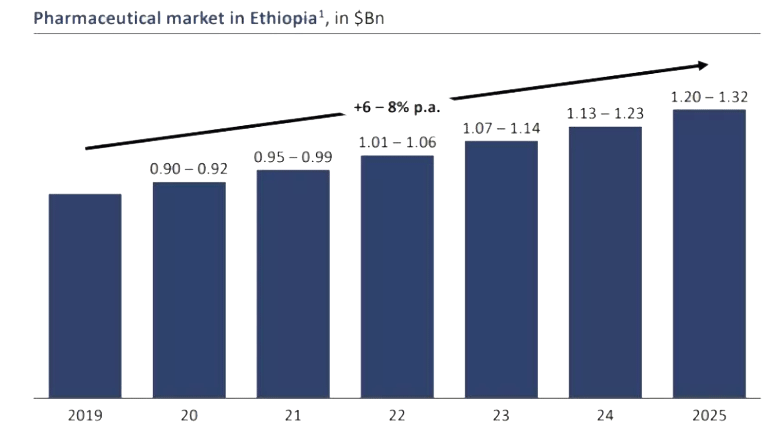
Importing of pharmaceuticals is the main source of covering the pharmaceutical demand in Ethiopia. Ethiopian Pharmaceutical Supply Agency (EPSA) and private suppliers are estimated to cover more than 80% of the demand. Of this, EPSA takes up the lion share by importing more than 70% of the total demand. Pharmaceutical importers in Ethiopia are not able to import and distribute medicines and/or supplies in the right quality and quantity at the right time. A wide range of challenges exist in the pharmaceutical supply chain system in Ethiopia that include legal, geographical, commercial and marketing or buyers‟ behavioral issues. Moreover, studies indicate that a significant portion of customers are not satisfied with the service provided by private importers as gauged by the five service quality dimensions (tangibility, reliability, responsiveness, assurance and empathy), reliability being the major dimension predicting satisfaction.
The import and distribution wing of MPSC after critically looking at the import landscape of the country came up with a resilient pharmaceutical supply chain system involving among others strategic and bulk purchasing, rational pricing, innovative delivery systems, tracking the distribution of pharmaceuticals, building strong relationship with source countries, and focusing on demand-driven import of pharmaceuticals.
MPSC is established to fill in these gaps and strive to bring about the highest level of customer satisfaction. The MPSC will conduct the importation, wholesale, and retail of pharmaceuticals, medical devices, diagnostic kits, and medical supplies by either establishing their own sales locations and pharmacies or franchising the opening of such locations throughout the country. MPSC not only procures health commodities, it also does the distribution for products procured by other organization like UNICEF, UNFPA,WHO and other partner organizations charging distribution fee.
Pharmaceutical Manufacturing
Overview of Current Pharmaceutical Manufacturing in Ethiopia
The pharmaceutical industry is responsible for the discovery, development, manufacturing and distribution of drugs, medications, chemical, reagent, and medical supplies. The market has experienced significant growth during the past two decades, and pharma revenues worldwide totaled 1.27 trillion U.S. dollars in 2020 when the market was valued at just 390 billion U.S. dollars in 2001. The industry is one of the major growth areas in Africa, expected to be worth $40 to $65 billion by 2020. This growth is caused by urbanization, healthcare capacity, and a better business environment. Indeed, some governments have been promoting more local production of drugs to reduce the need for imports.
Ethiopia’s pharmaceutical market grew to nearly $1 billion. The local market has been growing steadily at an average of 25% per year by 2020. With a population of more than 110 million, increased access to health services, and a growing middle-class, demand is projected to grow exponentially and reach 3.6 billion by 2030. Ethiopia currently imports 85% of its pharmaceutical needs and there is a growing domestic need for affordable pharmaceutical products to meet Ethiopia’s disease burden.
The development of the Ethiopian local pharmaceuticals manufacturing sub-sector has been very much limited in terms of production capacity, technology acquisition, creation of employment opportunity and investment. The local industry comprises 22 pharmaceuticals and medical supplies manufacturers, out of which 11 are involved in the manufacture of pharmaceutical products. Moreover, Africa only has two API manufacturers. There are no API manufacturers in Ethiopia.
Most of the local manufacturers are not compliant with international good manufacturing practice (GMP), and no single product has been prequalified by WHO. Although there are more than 200 importers of pharmaceutical products and medical consumables in Ethiopia, none of them have moved to the next level (Level 2) in the pharmaceutical value chain (Figure).
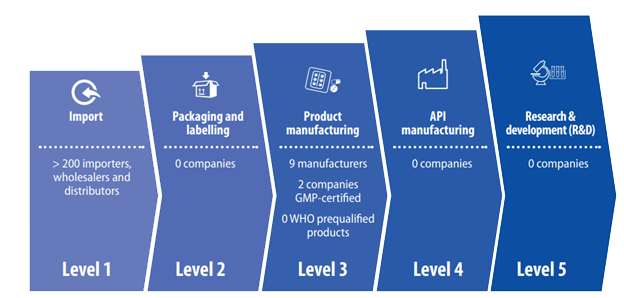
Figure: Pharmaceutical value chain: current situation in Ethiopia (Source: National strategy and plan of action for pharmaceutical manufacturing development in Ethiopia (2015–2025))
A wide range of challenges exist in the pharmaceutical supply chain system in Ethiopia that include legal, geographical, commercial and marketing or buyers‟ behavior issues. Moreover, studies indicate that a significant portion of customers are not satisfied with availability, sustainability, price, and quality of pharmaceuticals. According to Industrial Projects Studies of pharmaceutical formulation study, the annual demand for the six essential drug forms (Tablets, Capsules, Ampoules, Vials, Ointments and Syrups) is assumed to grow by 25%. The existing industries produce only generic and similar drugs in small quantities. The National Essential Drug List shows the availability requirement of 300 drugs in the country. Only about 90 of them are produced by the local manufacturers. Government and private clinics and hospitals in the country are coming up, and the demand for drugs and pharmaceutical products has increased.
In the Industrial Development Strategy of Ethiopia, it is clearly stated that the private sector is considered as the engine of the sector’s growth. Furthermore, special incentives are given to companies who are investing in the area and MPSC is there to seize this opportunity.
Momentous Pharmaceuticals Manufacturing
MPSC was established to navigate through the pharmaceutical value chain and become a modernized research-development based pharmaceutical industry. MPSC will produce raw materials, APIs, essential drugs, medical supplies, chemicals and reagents. Ethiopia being the second largest domestic market in Africa with over 110 million consumers, and growing consumer insurance awareness can be taken as the driving forces for MPSC’s vision to stand tall in the pharmaceutical arena so as to complement the efforts made to boost the country’s economy. MPSC will also work as A pharmaceutical manufacturing hub for sub-Saharan Africa. MPSC will show a tremendous progress in the pharmaceutical industry and lead the industry by example. The company will advance the quality of life for many patients and introduce fair pricing and distribution system within the country. It also aims to reward those who invest their money, time and idea in our company.
The global pharmaceuticals market is valued at over $1.27 trillion. The market was valued at just 390 billion U.S. dollars in 2001.
The Africa market for pharmaceuticals, valued over $65 billion
The Ethiopian pharmaceutical market is expected to reach $3.6 billion by 2030
The average return on investment for the pharmaceutical industry is around 10%
Our Partners











